Chimeric antigen receptor (CAR) T cell therapy is an increasingly prominent form of next-generation immunotherapy in both hematologic and solid tumor malignancies. Multiple CAR T products have received regulatory approval for use as treatment in relapsed and refractory leukemias and lymphomas. However, approximately half of these patients do not respond to therapy and production of CAR T cells is a difficult, expensive, and time-consuming process. Although much has been learned about the fundamental biology of CAR T cells over the past decade, we are still unable to accurately predict which patients will respond to therapy. In particular, few studies have specifically examined biological characteristics of manufactured CAR T cells and correlated these to relevant clinical outcomes.
In this study, we analyzed the lymphocyte populations and CAR T cells from patients enrolled in ACIT001/EXC002, a Canadian multi-centre phase Ib/II single-arm clinical trial of decentralized production of second-generation CD19/41BB/CD3z CAR T cells for treatment of multiply relapsed/refractory non-Hodgkin lymphoma (NHL) and acute lymphoblastic leukemia (ALL). CD3-positive T cells and CAR T products were comprehensively immunophenotyped by flow cytometry. Thecapacity for manufactured CAR T cells to kill tumor cells was assessed using an ex vivo cytotoxicity assay. CAR T cell immunophenotype and cytotoxic potential were correlated with clinical outcomes including response to therapy, progress-free survival (PFS) and incidence of serious adverse events.
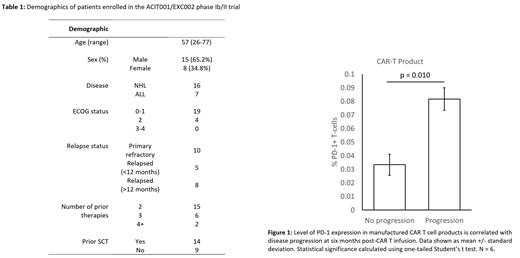
To date, 23 patients have been enrolled, of which 16 had an aggressive NHL while the remaining 7 had been diagnosed with B cell ALL. Mean age of participants at time of enrollment was 57 (range: 26-77) and 65.2% were male. CAR T product was manufactured using lentiviral transduction with a median transduction efficiency of 32.1% (range: 9.1% to 55.5%). A majority of patients achieved a complete response as evaluated by PET scan on day 28 post-CAR T cell infusion with only 3 patients showing disease progression, 1 patient achieving only partial response, and 3 patients being unevaluable. We were able to evaluate PFS at 6 months in 15 out of 23 patients, with 46.7% of patients experiencing progression and/or mortality within this period. Surprisingly, while CAR T cell cytotoxicity ex vivo trended higher in patients that achieved a complete metabolic response compared to those with partial response or disease progression, this did not reach statistical significance (p = 0.280). However, expression of T cell exhaustion markers such as PD-1 CAR T products was found to be significantly increased in non-responders (p = 0.010). Higher expression of exhaustion markers was also found to be associated with decreased CAR T persistence in vivo as measured by circulating CAR T cell levels at day 14 post-infusion. In our study cohort, CAR T products had either similar or significantly greater proportions of CD4+ as compared to CD8+ T cells. CAR T cells were found to express high levels of HLA-DR and CD127, however this did not correlate with cytotoxicity, response to therapy or PFS. Patient CAR T cells, regardless of the level of exhaustion marker expression, tended to be highly skewed towards the T effector memory subset, with almost no populations of naïve or memory T cells identified.
In this analysis of the impact of biological characteristics of CAR T cell products on patient outcomes, we found that CAR T cell exhaustion rather than cytotoxic activity is correlated with response to treatment and higher PFS at 6 months. Interestingly, the degree of patient T cell exhaustion had an impact on transduction efficiency but not the cytotoxic potential of the manufactured CAR T cell product. Taken together, our data indicate that CAR T cell exhaustion and persistence following infusion rather than cytotoxic potential is a key predictor for patient response to treatment. While our conclusions are currently limited by small sample size, more patients are presently being accrued to allow for more robust analysis in the future.
Disclosures
Sandhu:Gilead/Kite: Honoraria; Pfizer: Honoraria; Forus: Honoraria; Janssen: Honoraria; Celgene/BMS: Honoraria; Sanofi: Honoraria. Chu:AstraZeneca: Honoraria; BMS/Celgene: Honoraria, Research Funding; Gilead: Honoraria; Janssen: Honoraria; AbbVie: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Miltenyi: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal